Difference between revisions of "Sulphur or Gandhaka (गन्धकः)"
(adding content) |
|||
| Line 22: | Line 22: | ||
Rasajalanidhi<ref name=":2">Mookerjee, Bhudeb. (1984 Second Edition) ''Rasa-Jala-Nidhi or Ocean of Indian Chemistry, Medicine and Alchemy'', ''Vol. 2'' Varanasi: Srigokul Mudranalaya</ref> cites a few more following terms | Rasajalanidhi<ref name=":2">Mookerjee, Bhudeb. (1984 Second Edition) ''Rasa-Jala-Nidhi or Ocean of Indian Chemistry, Medicine and Alchemy'', ''Vol. 2'' Varanasi: Srigokul Mudranalaya</ref> cites a few more following terms | ||
| − | गन्धाश्मा गन्धको गन्धो गन्धी च गन्धिकों | + | गन्धाश्मा गन्धको गन्धो गन्धी च गन्धिकों वलिः। सौगन्धिकः सुगन्धिकः पामघ्नो गन्धमोदनः ॥ |
| − | + | शुल्वारिः पूतगन्धश्च कुष्ठारिर्दिव्यगन्धकः। सुगन्धी रसगन्धश्च कीटघ्नः क्रूरगन्धकः ॥ | |
| − | + | नवनीतस्तथा प्रोक्तो गन्धेशः शरभूमिजः। | |
| − | |||
| − | |||
| − | |||
| − | नवनीतस्तथा प्रोक्तो गन्धेशः | ||
== इतिहासः ॥ History == | == इतिहासः ॥ History == | ||
| Line 38: | Line 34: | ||
== स्वरूपम् ॥ Appearance == | == स्वरूपम् ॥ Appearance == | ||
| − | Gandhaka is available in both free and bound forms. Sulfur mixed with metals is available in pure form near volcanic mountains and at other places. Sulfur is found in the form of both sulphide and sulphate compounds. | + | Gandhaka is available in both free and bound forms. Sulfur mixed with metals is available in pure form near volcanic mountains and at other places. Sulfur is found in the form of both sulphide and sulphate compounds. |
== गन्धकखनिजानि ॥ Sulfur Minerals == | == गन्धकखनिजानि ॥ Sulfur Minerals == | ||
| − | Naturally Sulphur is available in the form of various minerals of sulphur. Gandhaka or Sulphur is extracted from these minerals. Some of the suphur minerals are listed below, | + | Naturally Sulphur is available in the form of various minerals of sulphur. Gandhaka or Sulphur is extracted from these minerals. Some of the suphur minerals are listed below,<ref name=":1" /> |
* Sulphide - in Sulphide form | * Sulphide - in Sulphide form | ||
| Line 58: | Line 54: | ||
== गन्धकस्य नैसर्गिकस्त्रोताः ॥ Natural sources of Sulphur == | == गन्धकस्य नैसर्गिकस्त्रोताः ॥ Natural sources of Sulphur == | ||
| − | Sulfur is also found in some organic substances like radish, onion, garlic, egg, etc. and in coal and crude petroleum products. These are the natural sources through which one can get organic form of sulphur. | + | Sulfur is also found in some organic substances like radish, onion, garlic, egg, etc. and in coal and crude petroleum products. These are the natural sources through which one can get organic form of sulphur.<ref name=":1" /> In cosmic abundance, sulfur ranks ninth among the elements, accounting for only one atom of every 20,000–30,000. Sulfur occurs in the uncombined state as well as in combination with other elements in rocks and minerals that are widely distributed, although it is classified among the minor constituents of Earth’s crust, in which its proportion is estimated to be between 0.03 and 0.06 percent. On the basis of the finding that certain meteorites contain about 12 percent sulfur, it has been suggested that deeper layers of Earth contain a much larger proportion. Seawater contains about 0.09 percent sulfur in the form of sulfate. In underground deposits of very pure sulfur that are present in domelike geologic structures, the sulfur is believed to have been formed by the action of bacteria upon the mineral anhydrite, in which sulfur is combined with oxygen and calcium. Deposits of sulfur in volcanic regions probably originated from gaseous hydrogen sulfide generated below the surface of Earth and transformed into sulfur by reaction with the oxygen in the air.<ref name=":0" /> |
| − | == प्राप्तिस्थानम् ॥ Place of | + | === प्राप्तिस्थानम् ॥ Place of Availability === |
Free sulfur is found in the Sicilian volcanic region of 'Italy', New Zealand, Japan, Spain, Texas etc. countries. It can also be found in countries like Russia, Japan, Burma, Iceland, America, Chile, Philippines, etc. In India, sulfur is found in abundance in Singhbhum district and Rohitas district of Jharkhand province, in Rajasthan, Kumaon and Assam. | Free sulfur is found in the Sicilian volcanic region of 'Italy', New Zealand, Japan, Spain, Texas etc. countries. It can also be found in countries like Russia, Japan, Burma, Iceland, America, Chile, Philippines, etc. In India, sulfur is found in abundance in Singhbhum district and Rohitas district of Jharkhand province, in Rajasthan, Kumaon and Assam. | ||
| Line 66: | Line 62: | ||
Ayurveda scholars have described various types of Gandhaka. It is classified on the basis of color, mode of consumption or utilization etc. Depending on its colour the supremacy of the gandhaka type and its value based on it has been decided. Gandhaka types are thus further also graded into good, better, best types. From a philosophical point of view, sulfur is the same but there are many differences of sulfur in shape, color etc. | Ayurveda scholars have described various types of Gandhaka. It is classified on the basis of color, mode of consumption or utilization etc. Depending on its colour the supremacy of the gandhaka type and its value based on it has been decided. Gandhaka types are thus further also graded into good, better, best types. From a philosophical point of view, sulfur is the same but there are many differences of sulfur in shape, color etc. | ||
| − | Four types of Sulphur based on colour<ref name=":1" /><ref name=":0" /> | + | Four types of Sulphur are categorized based on colour<ref name=":1" /><ref name=":0" /> |
| + | |||
| + | चतुर्धा गन्धको ज्ञेयो वर्णैः श्वेतादिभिः खलु । श्वेतोऽत्र खटिकाकारो लेपनाल्लोहमारणः ॥ | ||
| − | # '''Rakta''' - Red in color. Also known as Shukatundanibha i.e. of the color of Parrot's beak. This type is considered the best one and utilized chiefly in Dhatuvada (Metallurgy) | + | तथा चामलसारः स्यात् यो भावेत् पीतवर्णवान् । शुकपिच्छः स एव स्यात् श्रेष्ठो रसे रसायने ॥ |
| − | # '''Peeta''' - Yellow in color. Also known as aamlasara or Shukapichhanibha i.e of the color of tail of parrot. This type is commonly used in medicine. | + | |
| − | # '''Shweta''' - Also known as Khatika type which is white in color. It is of inferior quality. | + | रक्तश्च शुकतुण्डाख्यो धातुवादविधौ वारः । दुर्लभः कृष्णवर्णश्च स जरामृत्युनाशनः ॥ |
| − | # '''Krshna''' - It is said to be the rarest type of Gandhaka | + | # रक्तवर्णः ॥ '''Rakta''' - Red in color. Also known as Shukatundanibha i.e. of the color of Parrot's beak. This type is considered the best one and utilized chiefly in Dhatuvada (Metallurgy) |
| + | # पीतवर्णः ॥ '''Peeta''' - Yellow in color. Also known as aamlasara or Shukapichhanibha i.e of the color of tail of parrot. This type is commonly used in medicine. | ||
| + | # श्वेतवर्णः ॥ '''Shweta''' - Also known as Khatika type which is white in color. It is of inferior quality. | ||
| + | # कृष्णवर्णः ॥ '''Krshna''' - It is said to be the rarest type of Gandhaka | ||
Form of Gandhaka which is considered best for use in Medicine | Form of Gandhaka which is considered best for use in Medicine | ||
| Line 81: | Line 82: | ||
== Physical Properties of Sulphur == | == Physical Properties of Sulphur == | ||
| − | Sulfur is a yellow colored gem-shaped hard substance which shatters to pieces. It emits a special type of smell which becomes very intense in contact with fire. The gas emanating from it, called sulfur dioxide, is suffocating and gives shortness of breath. The melting point of sulfur is 115°C and it becomes thick at 230°C. On increasing the temperature, its color changes to the color of Nar. But it turns yellow again at higher temperatures above 500°C, its boiling point is 444°C. Its relative density is 2.06, it is insoluble in water.<ref name=":1">Mishra S. Textbook of Ayurvediya rasashstra. Chaukhamba Surbharati Prakashan. Edition Pg 348-54</ref> | + | Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. It reacts with all metals except gold and platinum, forming sulfides; it also forms compounds with several nonmetallic elements.<ref name=":0" /> |
| + | |||
| + | Sulfur is a yellow colored gem-shaped hard substance which shatters to pieces. It emits a special type of smell which becomes very intense in contact with fire. The gas emanating from it, called sulfur dioxide, is suffocating and gives shortness of breath. The melting point of sulfur is about 115°C and it becomes thick at 230°C. On increasing the temperature, its color changes to the color of Nar. But it turns yellow again at higher temperatures above 500°C, its boiling point is 444°C. Its relative density is 2.06, it is insoluble in water.<ref name=":1">Mishra S. Textbook of Ayurvediya rasashstra. Chaukhamba Surbharati Prakashan. Edition Pg 348-54</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
|+Element Properties<ref name=":0" /> | |+Element Properties<ref name=":0" /> | ||
| Line 90: | Line 93: | ||
|32.064 | |32.064 | ||
|- | |- | ||
| − | !melting point | + | ! colspan="2" |melting point |
| − | |||
|- | |- | ||
!rhombic | !rhombic | ||
| Line 102: | Line 104: | ||
|444.6 °C (832 °F) | |444.6 °C (832 °F) | ||
|- | |- | ||
| − | !density (at 20 °C [68 °F]) | + | ! colspan="2" |density (at 20 °C [68 °F]) |
| − | |||
|- | |- | ||
| − | !rhombic | + | !'''rhombic''' |
|2.07 grams/cm<sup>3</sup> | |2.07 grams/cm<sup>3</sup> | ||
|- | |- | ||
| − | !monoclinic | + | !'''monoclinic''' |
|1.96 grams/cm<sup>3</sup> | |1.96 grams/cm<sup>3</sup> | ||
|- | |- | ||
| − | !oxidation states | + | !'''oxidation states''' |
|−2, +4, +6 | |−2, +4, +6 | ||
|- | |- | ||
| Line 119: | Line 120: | ||
== Uses of Sulphur == | == Uses of Sulphur == | ||
| − | Sulphur is used for multiple purposes in different industries. Few common uses of sulphur apart from medicinal use are listed below, | + | Sulphur is used for multiple purposes in different industries. Few common uses of sulphur apart from medicinal use are listed below,<ref name=":1" /> |
* In making Sulfur Dioxide gas, from which Sulfuric Acid and Sulphide are made which are very useful for Metallurgy . | * In making Sulfur Dioxide gas, from which Sulfuric Acid and Sulphide are made which are very useful for Metallurgy . | ||
Revision as of 12:17, 7 October 2024
Gandhaka (Samskrit: गन्धकः) means Sulphur (also Sulfur) in English. It is a chemical element with atomic number 16 and chemical formula 'S'. Sulfur (S), nonmetallic chemical element belonging to the oxygen group (Group 16 [VIa] of the periodic table), is one of the most reactive of the elements. Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. It reacts with all metals except gold and platinum, forming sulfides; it also forms compounds with several nonmetallic elements. Millions of tons of sulfur are produced each year, mostly for the manufacture of sulfuric acid, which is widely used in industry.[1]
Though it is a non-metal, ancient Indian alchemists studied about this element at length, its ores, availability and properties and its role in curing physical decay, skin diseases and senility has been in use in Ayurveda. Texts such as Arthashastra give us extensive information about metals, non-metals, ores and their uses and their compounds.
परिचयः ॥ Introduction
In Ayurveda's Rasashastra (रसशास्त्रम्), sulphur is used widely in medicinal formulations. In Rasashastra (रसशास्त्रम्) treatises, various details about sulphur like appearance, types, purification, processing, use in medicinal formulations etc., have been discussed in depth. Although sulfa drugs are popularly used antibiotics and represent therapeutic use of Sulphur in Western medicine, the nature and form in which sulphur in utilized in Ayurvedic medicines is completely different and it has been in practice for thousands of years before western medicine used it as antibiotic.[2]
पर्यायाः ॥ Synonyms of Gandhaka used in Ayurveda
According to Mishra[2], the following are the synonyms used for sulphur.
- Gauripushpa (गौरीपुष्पः)
- Balivasa (बलिवसा)
- Lelitaka (लेलितक)
- Atigandha (अतिगन्धः)
- Kushthari (कुष्ठारिः)
- Kitaghna (कीटघ्नः)
- Navaneeta (नवनीतः)
- Shulbari (शुल्बारिः)
- Pamari (पामारिः)
- Bali (बलिः)
- Sugandha (सुगन्धः)
Rasajalanidhi[3] cites a few more following terms
गन्धाश्मा गन्धको गन्धो गन्धी च गन्धिकों वलिः। सौगन्धिकः सुगन्धिकः पामघ्नो गन्धमोदनः ॥
शुल्वारिः पूतगन्धश्च कुष्ठारिर्दिव्यगन्धकः। सुगन्धी रसगन्धश्च कीटघ्नः क्रूरगन्धकः ॥
नवनीतस्तथा प्रोक्तो गन्धेशः शरभूमिजः।
इतिहासः ॥ History
The knowledge of Indians about sulfur is very ancient. In the oldest and most popular treatise of Ayurveda Charaka Samhita (चरक संहिता) which was written 1200 years before Christ, sulfur has been used at many places for the treatment of many diseases. Its use has increased in texts written in later times. In the texts after the 8th century, Parada (पारदः Mercury) was mixed with Gandhaka and used in medicines and in Dhatuvada (धातुवाद) or metallurgical work. Since then till today no metal work is done without sulphur. The name Shulbari etc. is the symbol of Sulphur being the enemy of copper. If copper is heated by mixing sulphur, then copper gets destroyed.
Mercury is considered an element of Shiva, whereas sulphur, an element of Parvati and the product that was created from their union in asafoetida was called ras sindur, which was considered the essence for longevity.[4] In History of Hindu Chemistry, Sulphur has been mentioned as one of the eight uparasas, useful in operations of mercury.[5]
स्वरूपम् ॥ Appearance
Gandhaka is available in both free and bound forms. Sulfur mixed with metals is available in pure form near volcanic mountains and at other places. Sulfur is found in the form of both sulphide and sulphate compounds.
गन्धकखनिजानि ॥ Sulfur Minerals
Naturally Sulphur is available in the form of various minerals of sulphur. Gandhaka or Sulphur is extracted from these minerals. Some of the suphur minerals are listed below,[2]
- Sulphide - in Sulphide form
- Iron Pyrite Iron Pyrite (Fe S2)
- Copper Pyrite Copper Pyrite (Cu,S Fe, S)
- Galena (PbS)
- Cinnabar (Hg S)
- Hydrogen Sulfide Hydrogen Sulfide (H, S)
- Sulphate - In the form of Sulphate.
- Gypsum or calcium sulfate (Ca SO 2H, O)
- Heavy spar (Ba SO )
- Ferrous Sulphate (FeSO, 7H, O)
- Copper Sulphate (Cu SO, SHO)
गन्धकस्य नैसर्गिकस्त्रोताः ॥ Natural sources of Sulphur
Sulfur is also found in some organic substances like radish, onion, garlic, egg, etc. and in coal and crude petroleum products. These are the natural sources through which one can get organic form of sulphur.[2] In cosmic abundance, sulfur ranks ninth among the elements, accounting for only one atom of every 20,000–30,000. Sulfur occurs in the uncombined state as well as in combination with other elements in rocks and minerals that are widely distributed, although it is classified among the minor constituents of Earth’s crust, in which its proportion is estimated to be between 0.03 and 0.06 percent. On the basis of the finding that certain meteorites contain about 12 percent sulfur, it has been suggested that deeper layers of Earth contain a much larger proportion. Seawater contains about 0.09 percent sulfur in the form of sulfate. In underground deposits of very pure sulfur that are present in domelike geologic structures, the sulfur is believed to have been formed by the action of bacteria upon the mineral anhydrite, in which sulfur is combined with oxygen and calcium. Deposits of sulfur in volcanic regions probably originated from gaseous hydrogen sulfide generated below the surface of Earth and transformed into sulfur by reaction with the oxygen in the air.[1]
प्राप्तिस्थानम् ॥ Place of Availability
Free sulfur is found in the Sicilian volcanic region of 'Italy', New Zealand, Japan, Spain, Texas etc. countries. It can also be found in countries like Russia, Japan, Burma, Iceland, America, Chile, Philippines, etc. In India, sulfur is found in abundance in Singhbhum district and Rohitas district of Jharkhand province, in Rajasthan, Kumaon and Assam.
गन्धकभेदाः ॥ Sulfur Types
Ayurveda scholars have described various types of Gandhaka. It is classified on the basis of color, mode of consumption or utilization etc. Depending on its colour the supremacy of the gandhaka type and its value based on it has been decided. Gandhaka types are thus further also graded into good, better, best types. From a philosophical point of view, sulfur is the same but there are many differences of sulfur in shape, color etc.
Four types of Sulphur are categorized based on colour[2][1]
चतुर्धा गन्धको ज्ञेयो वर्णैः श्वेतादिभिः खलु । श्वेतोऽत्र खटिकाकारो लेपनाल्लोहमारणः ॥
तथा चामलसारः स्यात् यो भावेत् पीतवर्णवान् । शुकपिच्छः स एव स्यात् श्रेष्ठो रसे रसायने ॥
रक्तश्च शुकतुण्डाख्यो धातुवादविधौ वारः । दुर्लभः कृष्णवर्णश्च स जरामृत्युनाशनः ॥
- रक्तवर्णः ॥ Rakta - Red in color. Also known as Shukatundanibha i.e. of the color of Parrot's beak. This type is considered the best one and utilized chiefly in Dhatuvada (Metallurgy)
- पीतवर्णः ॥ Peeta - Yellow in color. Also known as aamlasara or Shukapichhanibha i.e of the color of tail of parrot. This type is commonly used in medicine.
- श्वेतवर्णः ॥ Shweta - Also known as Khatika type which is white in color. It is of inferior quality.
- कृष्णवर्णः ॥ Krshna - It is said to be the rarest type of Gandhaka
Form of Gandhaka which is considered best for use in Medicine
- Yellowish-green in color like parrot's tail,
- smooth,
- hard and
- gem-shaped sulfur is superior.
Physical Properties of Sulphur
Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. It reacts with all metals except gold and platinum, forming sulfides; it also forms compounds with several nonmetallic elements.[1]
Sulfur is a yellow colored gem-shaped hard substance which shatters to pieces. It emits a special type of smell which becomes very intense in contact with fire. The gas emanating from it, called sulfur dioxide, is suffocating and gives shortness of breath. The melting point of sulfur is about 115°C and it becomes thick at 230°C. On increasing the temperature, its color changes to the color of Nar. But it turns yellow again at higher temperatures above 500°C, its boiling point is 444°C. Its relative density is 2.06, it is insoluble in water.[2]
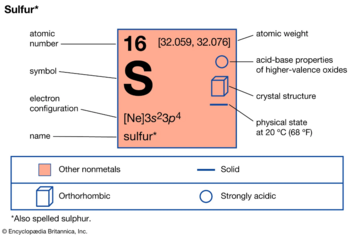
| atomic number | 16 |
|---|---|
| atomic weight | 32.064 |
| melting point | |
| rhombic | 112.8 °C (235 °F) |
| monoclinic | 119 °C (246 °F) |
| boiling point | 444.6 °C (832 °F) |
| density (at 20 °C [68 °F]) | |
| rhombic | 2.07 grams/cm3 |
| monoclinic | 1.96 grams/cm3 |
| oxidation states | −2, +4, +6 |
| electron configuration | 1s22s22p63s23p4 |
Uses of Sulphur
Sulphur is used for multiple purposes in different industries. Few common uses of sulphur apart from medicinal use are listed below,[2]
- In making Sulfur Dioxide gas, from which Sulfuric Acid and Sulphide are made which are very useful for Metallurgy .
- Carbon dioxide gas in fireworks, matches, and gunpowder etc.
- Useful in vulcanizing rubber.
- Useful in purifying metals and making fertilizers.
Doshas / Impurities in Sulphur
गन्धे मलद्वयं दृष्टं शिलाचूर्णं विषं तथा । शोधितव्यस्ततो यत्नादभिज्ञेन यथाविधि॥[3]
There are two types of impurities in sulphur
- शिलाचूर्णम् ॥ Shila Churna (physical impurities like clay, sand etc) and
- विषम् ॥ Visha Dosha (chemical impurities like arsenic, lead etc)
Therefore, it should be purified by a skilled physician with care. Impure sulphur gives rise to leprosy, giddiness, diseases due to an excess of pitta, loss of beauty, happiness, strength and semen.
Harmful effects of consuming impure Sulphur
Ayurveda advocates use of any mineral or metal only after its thorough purification known as Shodhana. It is applicable for Gandhaka as well. Rasashastra branch of Ayurveda provides various methods to purify and process Gandhaka before using it in the medicinal formulations. If the appropriate methods of processing Gandhaka are not used or they are skipped and such Gandhaka/Sulphur is used in the medicinal formulations then it can certainly cause some ill effects. Ayurveda acharyas have deeply studied these effects and described those to make people aware and cautious. There is tale about origin of Sulphur and its resultant effects
Leprosy by consuming impure sulphur- Anger
And produces gall disease. Destroys form, semen, strength and happiness. Pure sulfur should always be used.
If sulfur is not purified properly while consuming it, etc. Halahal kills a person like using poison. Because sulfur originated at the time of Gandhak Utpa time (according to the context of earlier reading, sulfur was generated at the time of churning of the ocean; had been found in sulfur. Therefore, improper use works like poison.
By looking at Amlasar Gandhak, its toxicity etc. defects are not visible, these defects are visible in the form of poison on the person's body after use. That's why it is known as poisoning. which is experiential. Sulfur
Properties of pure sulfur
Pure sulfur is leprosy killer. Makes immortal. Yet old age and death are destroyers. There is a fire lamp, it is very hot. increase in body semen
It is Rata. ,
Pure sulfur is very chemical, it is sweet but it is bitter and hot in cooking. Kandu, Leprosy, Visarp, Dadru killer and Jathragni is illuminating, digestive, common killer and absorbent, antidote, gives power to mercury, anthelmintic. Kimdhikan-Rasoparsa There is no other substance greater than sulfur in ordinary juices. ,
Dosage of pure gandhak for internal use
Pure sulfur should be given from 1 Ratti to 8 Ratti. ,
Remedy for Sulfur disorder
If any disorder arises in the body after consuming Sulphur, then drinking 100 grams of Ghoghrit and Mishri in 1 liter of milk for 2 weeks calms down the pain caused by Sulfur disorder.
At the time of consumption of gandhak, one should eat diet
wild animals-birds and Chaag meat. Cow's milk, cow's ghee, wheat, rice, sandhav, sugar candy, clean and pure cool water are the diet.
Unhealthy
Excessive salt, acid, bitter, diarrhea, vegetable, (leaf vegetable) bidal (pulse)
Kshar and Kanji should not be consumed. One should not board a fast-moving vehicle and consume women.
Some of the main formulations of Ayurvedic medicine made from pure sulfur-
- Arshakuthar ras
- Rasparpati
- Rasindoor
- Kanakasunderaras
- Jayamangalras
- Vijayabhairavaras
- Trisunderaras
- Panchamritparpati
- Samirpannagaras
- Saubhagyavati
- Mrityunjayavati
- Mahajvarankusharas
- Hanspotli
Sulpha allergies
Modern medicine has brought a new revolution in the medical world by inventing medicines called "Sulfadrug" in "Allopathy".
Sulfur is useful in killing bacteria and destroying fungus.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Brasted, R. C.. "sulfur." Encyclopedia Britannica, September 14, 2024. https://www.britannica.com/science/sulfur.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Mishra S. Textbook of Ayurvediya rasashstra. Chaukhamba Surbharati Prakashan. Edition Pg 348-54
- ↑ 3.0 3.1 Mookerjee, Bhudeb. (1984 Second Edition) Rasa-Jala-Nidhi or Ocean of Indian Chemistry, Medicine and Alchemy, Vol. 2 Varanasi: Srigokul Mudranalaya
- ↑ Soni, Suresh. India's Glorious Scientific Tradition.
- ↑ Ray, Prafulla Chandra. (1903) A history of Hindu Chemistry, from the earliest times to the middle of the sixteenth century A.D., Vol. 1. Calcutta: The Bengal Chemical & Pharmaceutical Works, Ltd